clinical trial malaysia
Clinical Trials are a process to determine the effectiveness and dangers of new medicines and other related medical practices for targeted patients. EU Clinical Trials Registry.
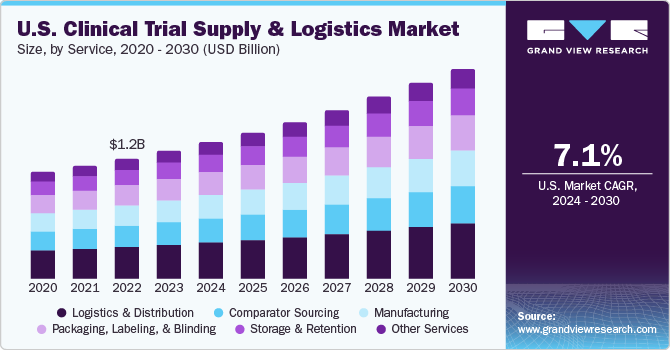
Clinical Trial Supply And Logistics Market Report 2028
Besides early phase clinical research we also conduct bioavailability and bioequivalence studies for pharmaceutical industries in accordance to local and international standards.
. Overview of GCP and Clinical Research in Malaysia. The current composition of the NCCR was established to ensure that it becomes visionary and pro-active in driving the development of clinical research in the country. Know the risks and potential benefits of clinical studies and talk to your health care provider before participating.
DEFINITION This Guideline adopts the following definitions. The health spending as a share of Gross Domestic Product GDP for the same period ranged from 295 per cent to 453 per cent of GDP. A properly planned and executed clinical trial is a powerful experimental technique for assessing the effectiveness of an intervention.
Malaysian investigators have over the past decade been involved in major clinical outcome trials which were subsequently published in major medical journals. For further information please contact the Principal Investigator. Before applying for a CTC an applicant should first receive approval from the hospitals Ethics Committee EC.
A The 2007 directive requiring all ethics committees that approve clinical trials in Malaysia to be registered with the Malaysian Drug Control Authority DCA a body established under the Regulations to regulate the quality safety and efficacy of. Listing a study does not mean it has been evaluated by the US. Hong Kong Indonesia Malaysia the Philippines Singapore Taiwan Thailand and Vietnam.
31 Clinical Trial - in which the objective of the trialresearch is of essentially diagnostic or therapeutic value to the patient. Oncology came in second in 2021 with 33 new studies. Phase I Phase I includes trials to assess the safety and effects of the medicines on body systems.
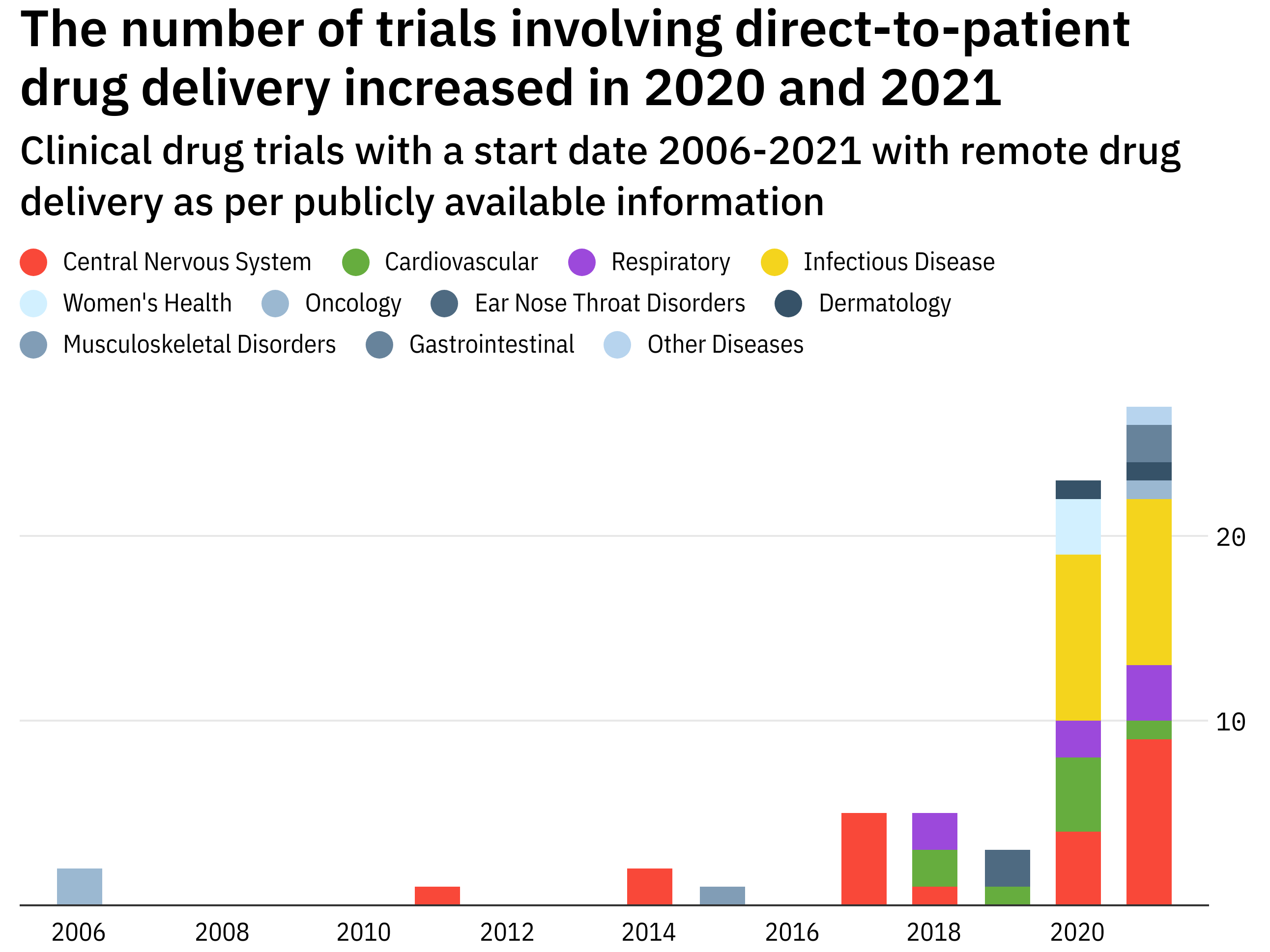
Last year infectious diseases topped new sponsored clinical research in Malaysia with 40 studies reversing the trend since 2012 when oncology comprised the majority of studies involving novel therapies. 712A GUIDE TO CONDUCTING CLINICAL TRIALS IN MALAYSIA Fig 2. Research and Clinical Trials Malaysian Oncological Society Healthcare Professionals Research and Clinical Trials The following are Industry Sponsored Clinical Research ISR currently conducted at the various centres.
More importantly their contributions have been translated into improved patient care and outcomes and underscore the importance of evidence-based practice. The highest value contracted last year was worth RM226 million. In most cases the smaller Asian countries will not require local clinical studies and will accept foreign clinical trial data during the registration process for both medical devices and pharmaceuticals.
A listing of Kuala Lumpur Malaysia clinical trials actively recruiting patients volunteers. Malaysia is George Clinicals Southeast Asian hub where we cover neighboring countries of Singapore Indonesia and the Philippines accessing a population of almost 400 million multi-ethnic people. International Council of Harmonization ICH.
EDC query edit and audit trails with real-time analytics dashboards across all devices. IMS Health and Quintiles are now IQVIA a world leader in using data technology advanced analytics and expertise to help customers drive healthcare -. Ad Leading provider of trial services virtual and hybrid sites and DCT software platform.
General Clinical Trial. To register new drugs applicants must also. Key features of the Malaysian clinical trial landscape include streamlined submission and regulatory processes in English quick start-up times a strong network of experienced KOLs and PIs and supportive Government policies all of which have made Malaysia a preferred clinical trial destination.
Obtaining EC approval generally takes 4 to 6 weeks. What is the regulatory authority with oversight for clinical trial in Malaysia. The Total Health Expenditure THE for Malaysia during 1997-2013 ranged from RM8303 million in 1997 to RM44748 million in 2013.
Within 10 mi 15 km Filter By Advanced I amhavehad ct scan treatment regimen chest x-ray gaa gene movement disorder more I am looking for ergocalciferol etelcalcetide dasatinib pemetrexed adalimumab more Found 202 clinical trials By relevance. For more details please click here. EDC query edit and audit trails with real-time analytics dashboards across all devices.
Under Regulation 36B of the Pharmacy and Poisons Regulations a certificate for Clinical TrialMedicinal Test CTC is required before conducting a clinical trial. Malaysia has a single regulatory authority the National Pharmaceutical Control Bureau NPCB. Ad Leading provider of trial services virtual and hybrid sites and DCT software platform.
US Clinical Trials Registry. Ethics and the IRBIEC. A clinical trial conducted according to a single protocol but at more than one site and therefore carried out by more than one investigator.
The clinical trials are divided into four different clinical phases. ABDOMINAL PELVIC BLADDER CANCER BREAST CANCER. George Clinical In Malaysia.
DAY 1 Virtual Session. National Committee for Clinical Research A committee established for the purpose of coordinating and promoting clinical research in Malaysia chaired by the Director General of Health Ministry of Health. On August 2020 the NPRA of Malaysia has updated a document intended to guide the applicant in making Clinical Trial Import Licence CTIL and Clinical Trial Exemption CTX applications to NPRA and reporting to NPRA upon the completion of the clinical trial.
Singapore is a recognized destination for clinical trials yet other countries are fast emerging as more. List of Clinical Research Organizations in Malaysia Featured CROs in Malaysia IQVIA. The committee is made up of member representatives and experts from the Ministry of Health various national Universities the Malaysian Pharmaceutical Society the Pharmaceutical.
The Centre for Investigational New Product is the unit in charge. HCV Self-testing in Malaysia The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. GCP Compared to Malaysian Guideline for GCP.
Centre for Clinical Trial CCT was established in 2010 to conduct and support early and late phase clinical research in Malaysia.
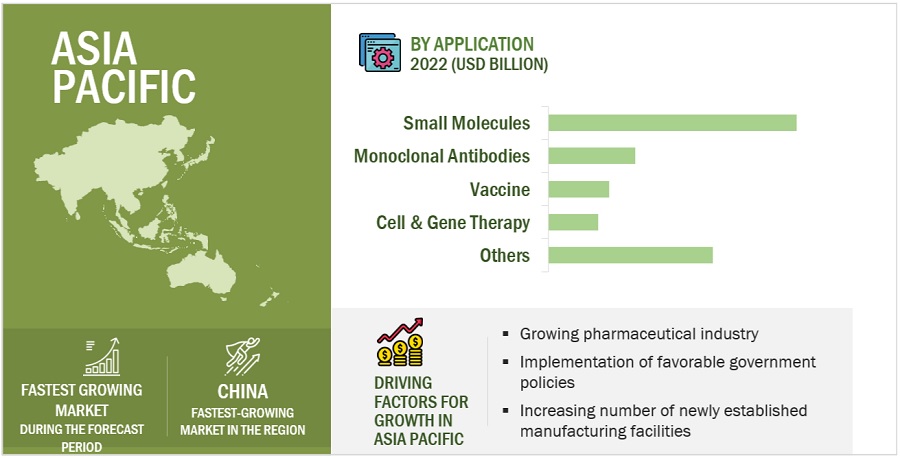
Clinical Trials Market Size Share 2022 2026 Marketsandmarkets
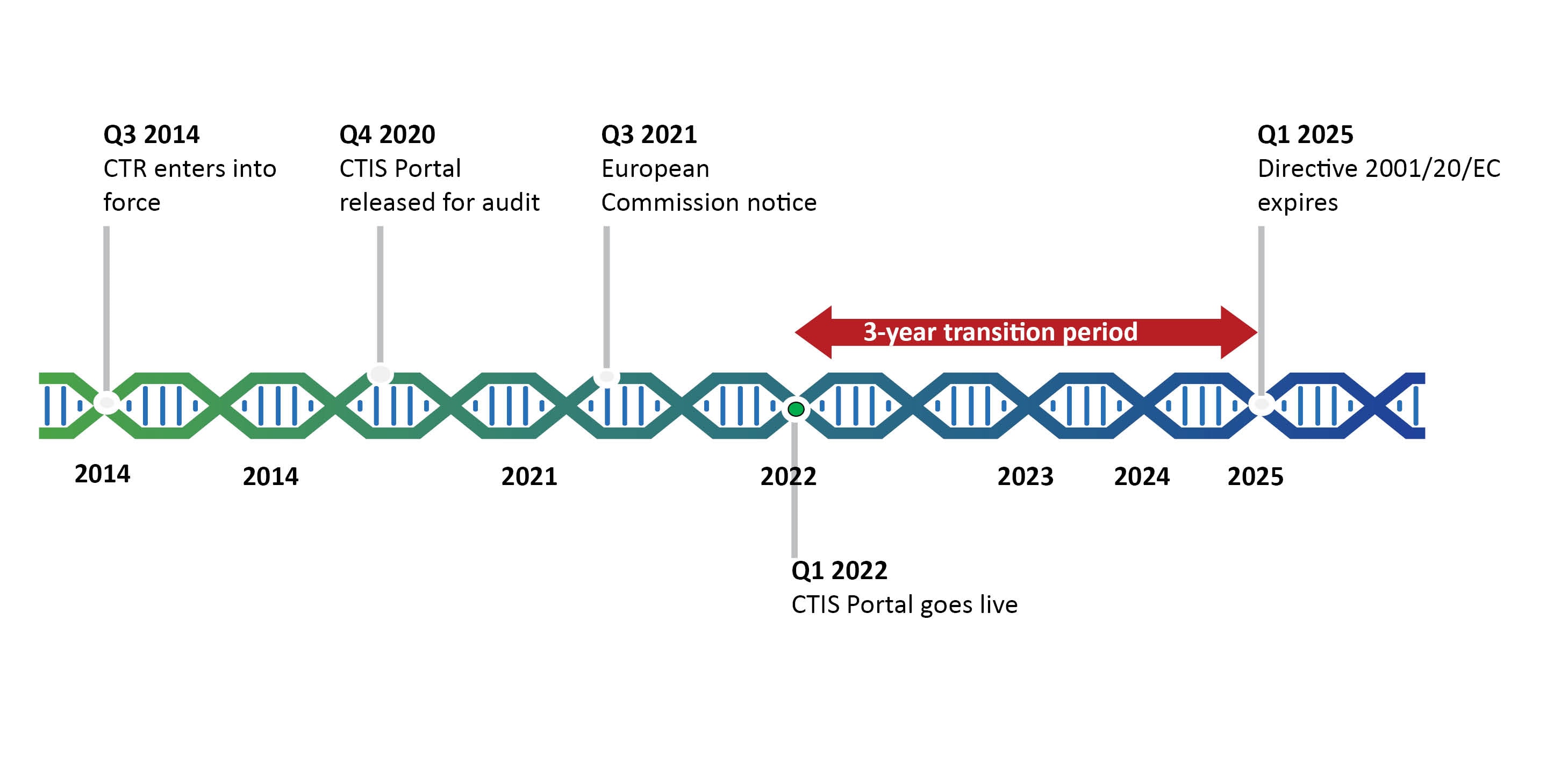
Introduction To The Clinical Trials Regulation Deloitte Netherlands

National Medical Research Register

Home Clinical Update In Covid 19
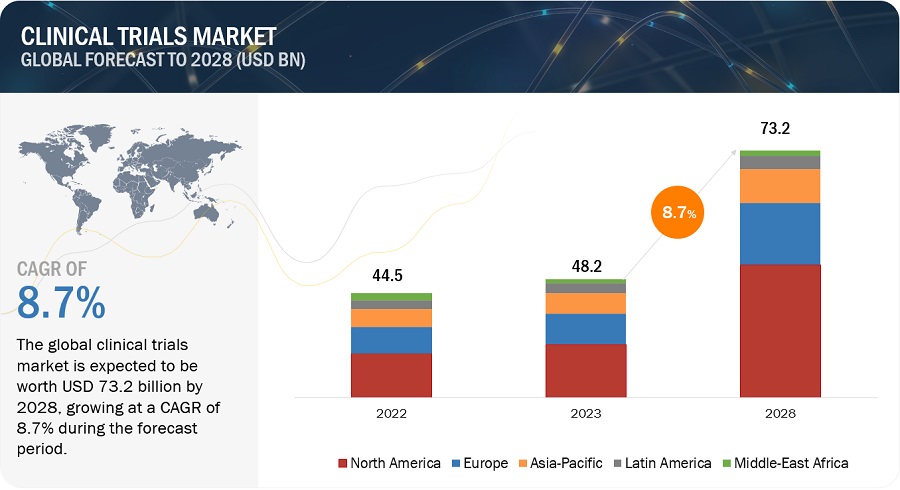
Clinical Trials Market Size Share 2022 2026 Marketsandmarkets
Why Asia Pacific Is The Next Frontier For Decentralised Clinical Trials Clinical Trials Arena
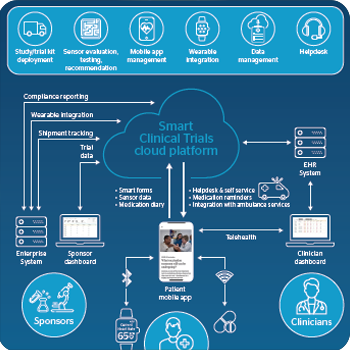
Accelerating Clinical Trials And Reducing Costs With Digital Technologies Atos

Direct To Patient The Rocky Road To Remote Drug Delivery In Clinical Trials

Ministry Of Health Malaysia And Drugs For Neglected Diseases Initiative Combine Forces To Lead The Battle Against Dengue Dndi

Cancer Research Malaysia Linkedin

The Future Of Clinical Trials In A Post Covid World Pharmaceutical Technology
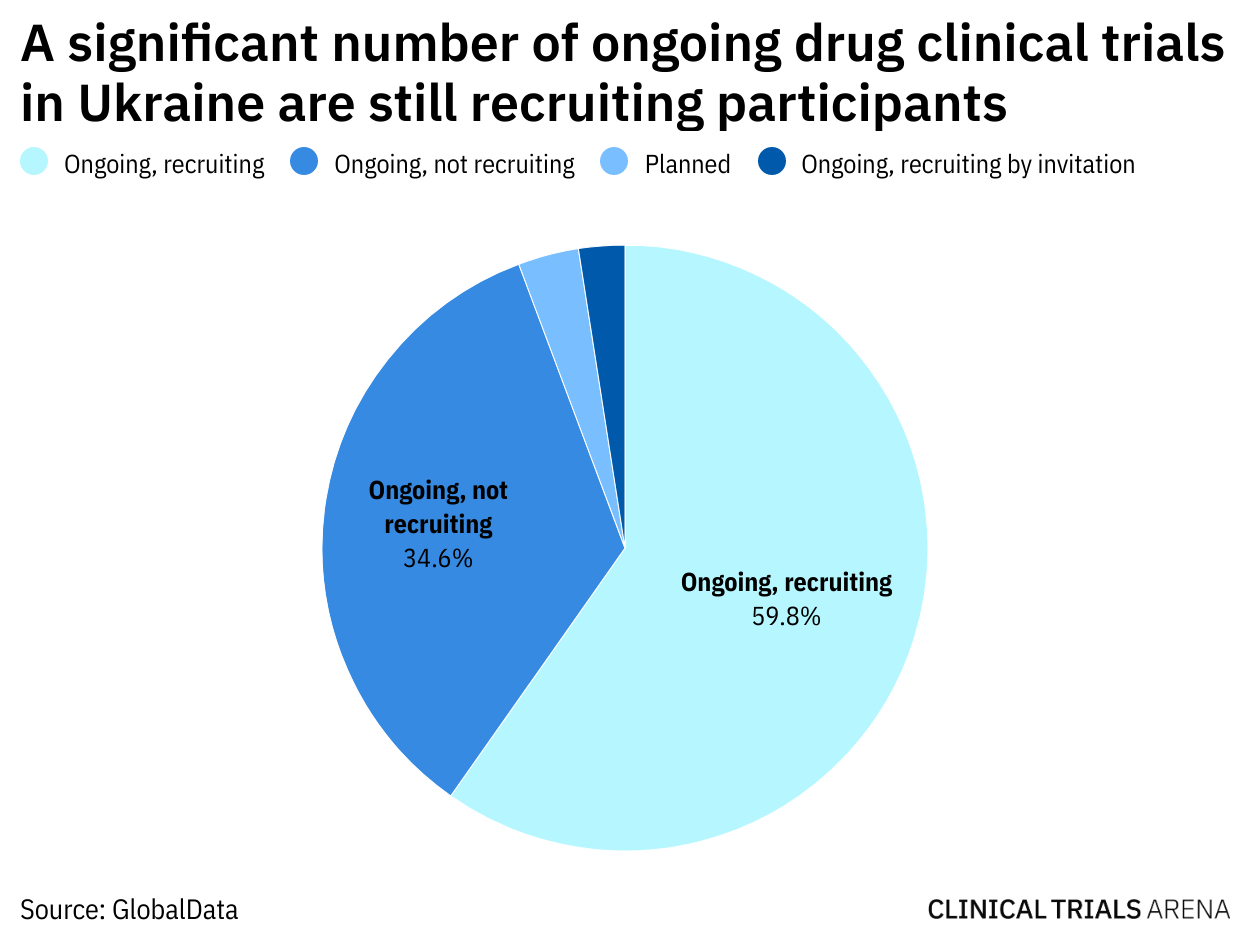
Ukraine Industry Sponsored Clinical Trial Development At Risk

Contract Research Organization Cro Malaysia Novotech Cro

Home Clinical Update In Covid 19

Introduction To The Clinical Trials Regulation Deloitte Netherlands
Encouraging Results From Clinical Trials Of Two Male Contraceptive Pills Technology Networks

Institute Of Clinical Research Linkedin

0 Response to "clinical trial malaysia"
Post a Comment